Abstract
Introduction Infectious complications contribute significantly to treatment-related morbidity after allogeneic hematopoietic stem cell transplant (HSCT). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a novel coronavirus that can result in significant lung damage with high mortality rate. On January 30, 2020, the World Health Organization declared that the outbreak of novel coronavirus constitutes a Public Health Emergency of International Concern. In response, various infection prevention measures were adopted by the public and health care facilities. Here we evaluate the incidence and clinical outcome of common upper respiratory virus as well as SARS-CoV-2 infection during COVID-19 pandemic in patients who newly underwent allogeneic bone marrow transplant.
Methods We conducted a retrospective review of the total number of seasonal respiratory viral panel (RVP) PCR (testing for influenza A, influenza B, respiratory syncytial virus, parainfluenza viruses, seasonal corona virus, human rhinovirus, adenovirus, human metapneumovirus) and SARS-CoV-2 PCR testing on nasopharyngeal swab performed between each flu reason (October to March) from 2017-2022. Respiratory viral infection rule out was commonly performed to evaluate patient symptoms or as pre-admission/procedure clearance. We used the flu season of 2017-2018, and 2018-2019 as historical control. To highlight the most vulnerable post-transplant population, we targeted the study on patients who were within 1-year post-allogeneic bone marrow transplant.
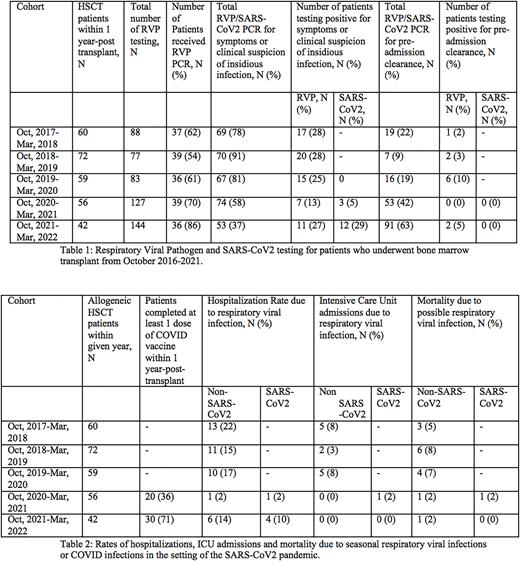
Results A total of 60, 72, 59, 56 and 42 patients underwent allogeneic bone marrow transplant at our institution in 2017, 2018, 2019, 2020 and 2021 respectively (table 1). In 2017-2018, 2018-2019, 2019-2020, 2020-2021, and 2021-2022, there were 37 (62%), 39 (54%), 36 (61%), 39 (70%) and 36 (86%) patients received respiratory viral testing with 69 (78%), 70 (91%), 67 (81%), 74 (58%), and 53 (37%) total testing conducted due to concerning symptoms, respectively. Seventeen (28%), 20 (28%), 15 (25%), 7 (13%), and 11 (27%) symptomatic patients tested positive for seasonal respiratory viruses in 2017-2018, 2018-2019, 2019-2020, 2020-2021, and 2021-2022, respectively. In 2020-2021 and 2021-2022, 3 (5%) and 12 (29%) symptomatic patients tested positive for SARS-CoV2, respectively. Of testing conducted for pre-procedure, admission or surveillance reasons, 1 (2%), 2 (3%), 6 (10%), 2 (5%) patients tested positive for seasonal respiratory viruses in 2017-2018, 2018-2019, 2019-2020, and 2021-2022, respectively. No patient tested positive for COVID on pre-procedure testing.
Regarding severity of respiratory virus infection, 13 (22%), 11 (15%), 10 (17%), 1 (2%), and 6 (14%) patients were hospitalized due to seasonal respiratory viral infections in 2017-2018, 2018-2019, 2019-2020, 2020-2021, and 2021-2022, respectively. In 2020-2021 and 2021-2022, 1 (2%) and 4 (10%) patients were hospitalized due to a COVID-19 infection. Considering intensive care unit (ICU) admissions due to respiratory virus infection, 5 (8%), 2 (3%), and 5 (8%) patients were admitted to the ICU in 2017-2018-, 2018-2019, and 2019-2020, respectively. 1 (2%) patient was admitted to the ICU for COVID 19 infection in 2020-2021. In terms of mortality, seasonal respiratory viral infections contributed to mortality in 3 (5%), 6 (8%), 4 (7%), 1 (2%) and 1 (2%) patients in 2017-2018, 2018-2019, 2019-2020, 2020-2021, and 2021-2022, respectively. COVID infections resulted in mortality for 1 (2%) patient in 2020-2021 (table 2).
Conclusion In this single institution retrospective study from 2017-2022, we observed a consistent incidence of respiratory virus infection prior to the SARS-CoV2 pandemic from 2017 to the beginning of 2020. The low incidence of seasonal respiratory virus infection in 2020-2021 flu season was likely due to the strict infection prevention measure and isolation recommendation. With increasing rate of vaccination, less stringent public health infection control policy in 2021-2022 flu season, a drastic increase in symptomatic RVP and SARS-CoV2 testing was noted. During the same time frame, the incidence of respiratory virus infection/hospitalization also increased significantly. The low mortality of SARS-CoV2 infection in 2021-2022 was possibly a result of high rate of vaccination against SARS-CoV2, available anti-viral therapy, and less virulent SARS-CoV2 variants.
Disclosures
Schiller:Onconova: Research Funding; AstraZeneca: Honoraria; Cellerant: Research Funding; Actinium: Research Funding; Agios: Consultancy, Honoraria; Astellas: Research Funding, Speakers Bureau; Jazz: Consultancy; Forma: Research Funding; Genentech-Roche: Research Funding; Cellectis: Research Funding; Deciphera: Research Funding; Glycomimetics: Research Funding; AVM Biopharma: Research Funding; Arog: Research Funding; CTI: Research Funding; Janssen: Research Funding; Sellas: Research Funding; Millennium: Research Funding; Samus: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Actuate: Research Funding; Cyclacel: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Deltafly: Research Funding; AltruBio: Research Funding; AbbVie: Research Funding, Speakers Bureau; Novartis: Honoraria, Other: Speaker fees, Research Funding; Ono Pharma: Honoraria; Amgen: Current equity holder in publicly-traded company, Honoraria; Karyopharm: Research Funding, Speakers Bureau; PreCOG LLC: Research Funding; Gamida: Research Funding; Mateon: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Daiichi-Sankyo: Research Funding; Medimmune: Research Funding; Geron: Research Funding; Gilead: Research Funding; Pfizer: Research Funding; Trovagen: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Stemline: Research Funding; Constellation: Research Funding; Regimmune: Research Funding; Stemline: Speakers Bureau; Johnson & Johnson: Current equity holder in publicly-traded company; FujiFilm: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal